What is the OHF Patient Registry?

By Joining the OHF Patient Registry:
- You can help change the future of treatment,
- Accelerate breakthroughs in treatment,
- And move us closer to a world without hyperoxaluria.
Your Journey. Your Health. Your Future.
The OHF Patient Registry is more than just data—it tells the story of people living with hyperoxaluria and helps shape the future of care. By sharing your or your child’s health information, you help doctors and researchers:
- Develop care guidelines
- Support care teams in providing the best care for patients with hyperoxaluria
- Track outcomes of current therapies and design future clinical trials.
Your participation drives progress—improving care, advancing new treatments, and making a real difference for everyone affected by hyperoxaluria.
Your privacy is protected. Any health information you share is stored securely and used only in a way that cannot identify you personally. This allows researchers to study hyperoxaluria while keeping your personal details private.
Sign Me Up, Today
Clinical Trials
Patients and caregivers play a vital role in advancing rare disease research. With conditions like hyperoxaluria, your participation in clinical trials helps researchers develop new treatments and bring them to patients who need them most.
Why participate in a clinical trial?
Joining a clinical trial can:
- Give you early access to new treatments not yet available to the public
- Provide expert care at leading healthcare facilities
- Let you take an active role in your own or your child’s healthcare
- Contribute to medical discoveries that could help others and change lives
How do clinical trials work?
Doctors will guide you through the process, explaining what to expect, including potential risks and benefits. Every study follows strict safety rules to keep participants protected. Before the trial begins, doctors may perform tests to understand your or your child’s health and will answer any questions you have. You can ask questions at any time and can leave the study whenever you choose.
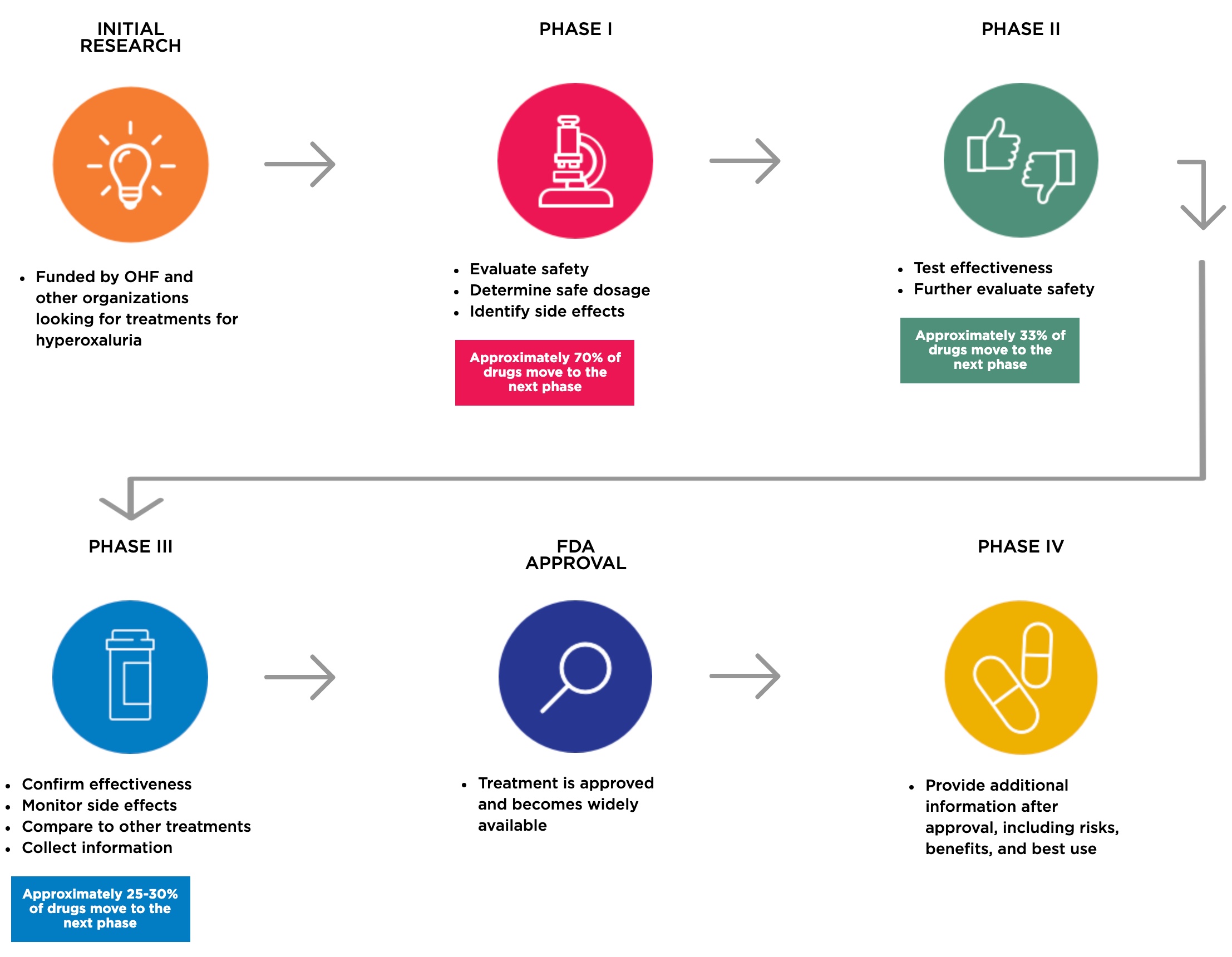
What are phases in a clinical trial?
Clinical trials are conducted in phases, each designed to answer specific questions while keeping participants as safe as possible. Most new treatments go through three or more phases before they are considered safe and effective for wider use.
Where can I find a clinical trial?
For information on hyperoxaluria studies, visit ClinicalTrials.gov, a trusted database of publicly and privately supported clinical studies from around the world.
Drug Development
Developing new treatments is a long and complex process that relies on collaboration between many experts. The Oxalosis and Hyperoxaluria Foundation is proud to play a key role in advancing promising therapies, working closely with researchers, companies, and the patient community to make new treatment options possible.
Nearly every treatment in development today reflects this teamwork and the contributions of patients, families, and caregivers.
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | To Patients |
Alnylam (Oxlumo— Lumasiran)
Primary Hyperoxaluria Type 1
To Patients
Arbor Biotechnologies (—)
Primary Hyperoxaluria
Preclinical
Biocodex (Stiripentol)
Primary Hyperoxaluria
Phase 3
Novo Nordisk (Rivfloza— Nedosiran)
Primary Hyperoxaluria Type 1
To Patients
Meta Pharmaceuticals (META-1i-PH)
Primary Hyperoxaluria
Preclinical
YolTech (Yolt-203)
Primary Hyperoxaluria
Phase 1
For Researchers
The OHF International Hyperoxaluria Workshop is the world’s largest gathering focused on this ultra-rare disease. Leading scientists, clinicians, and stakeholders come together to share breakthroughs, explore trends, and advance research that informs better care for people living with hyperoxaluria. Stay tuned for updates on the 2026 workshop!
Learn More About Grants & Funding
The OHF proudly funds innovative research into the causes and treatment of hyperoxaluria. We support scientists whose work is paving the way for better therapies and, ultimately, a cure.